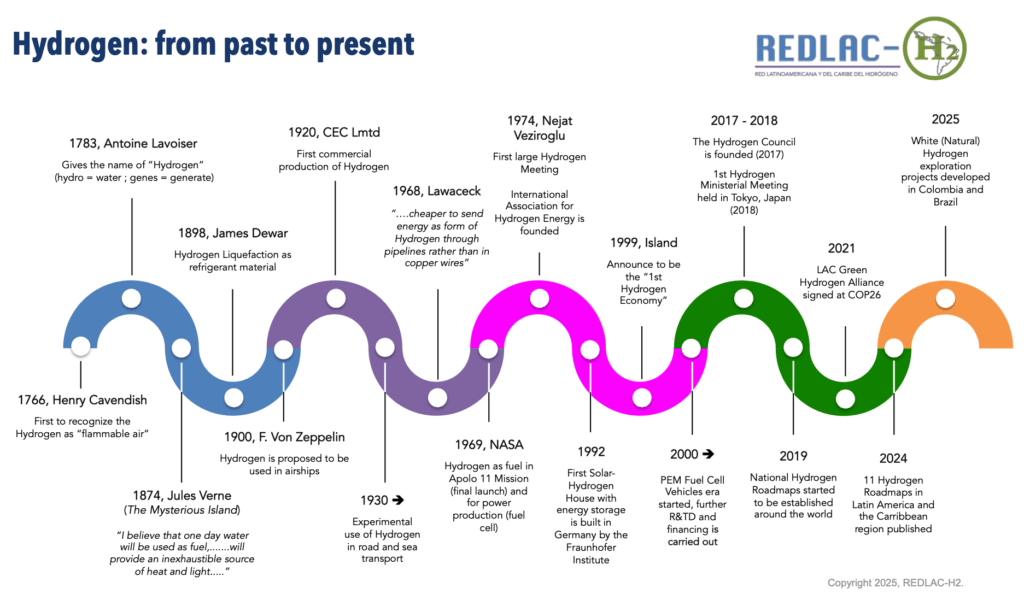
The Hydrogen
Hydrogen (H+) is the lightest chemical element that exists, and it occurs in a molecular state, forming the diatomic gas (H2) under normal conditions; which strictly speaking should be identified as Di-Hydrogen.
Hydrogen constitutes approximately 75% of the visible matter in the Universe and is responsible for a large amount of chemical energy in the form of electromagnetic radiation emitted by stars, due to nuclear fusion between hydrogen nuclei to transform into helium. This is the case of our star “The Sun”, whose energy gives us the light and heat necessary for life. On our planet Earth, Hydrogen is not found naturally as it is part of chemical compounds such as hydrocarbons or water, and different transformation processes are necessary for its production, which is used primarily in petroleum refining and the chemical industry (fertilizers and ammonia). By itself, hydrogen gas (H2) is not considered a primary energy source, but an energy vector with enormous potential for use in strategic sectors such as energy and transport, and the transformation industry (iron & steel, cement, mining, etc.).

Photo: Credits from NASA (nasa.gov), The Universe, Hubble Telescope image.
” I believe that someday water will be a fuel, that hydrogen and oxygen…..
will provide an inexhaustible source of heat and light…..”
Jules Verne, The Mysterious Island, 1874
Hydrogen is positioned as the main accelerator for the decarbonization of economies around the world, since it does not emit pollutants when is used directly for combustion or in fuel cells, as it is a “Net-Zero Emissions” carbon carrier.
The colors of Hydrogen



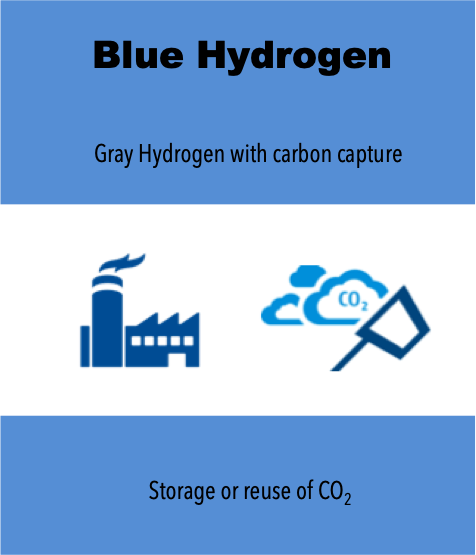


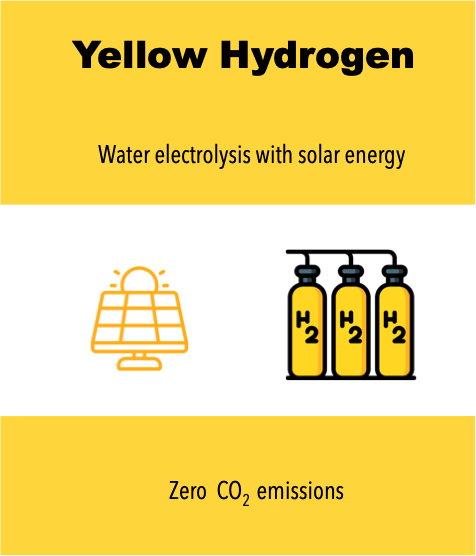


The most widely produced type of hydrogen worldwide is Gray Hydrogen, which is generated using natural gas (methane) through Steam Methane Reforming (SMR) technology. However, this process results in high CO₂ emissions. Gray Hydrogen is primarily used in oil refining for gasoline production and in the petrochemical industry for the production of ammonia and fertilizers. If the CO₂ emissions from this process are captured and stored using Carbon Capture and Storage (CCS) technologies, the hydrogen is then classified as Blue Hydrogen. However, the trend toward producing clean hydrogen (with zero CO₂ emissions) is rapidly accelerating. Water electrolysis technology—powered by renewable electricity—is gaining the most attention. This pathway gives rise to Yellow Hydrogen, Pink Hydrogen, and Green Hydrogen, though all currently come at a high cost (around 7–8 USD/kg H₂).
Alternative hydrogen production methods are also emerging under the Circular Economy concept, such as using residual lithium from batteries through its chemical reaction with water: (2Li(s) + 2H₂O(l) → H₂(g) + 2LiOH(aq)). Hydrogen produced this way is classified as Silver Hydrogen.
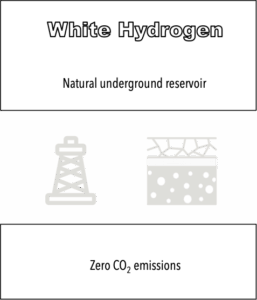
New frontiers in hydrogen production are also emerging, such as the exploration of Natural Hydrogen (or White Hydrogen), which is naturally occurring underground. There is significant global interest in its production due to its low cost (around 1–2 USD/kg H₂).

Use of Hydrogen

Energy.
Hydrogen is widely regarded as the most important future energy vector for electricity generation and energy security, due to its versatility in storage. When there is surplus electricity from renewable sources, hydrogen gas can be produced through the electrolysis of water and subsequently stored and/or injected into natural gas grids—this process, known as Power-to-Gas (P2G), enables the conversion of excess energy into a storable and transportable fuel.

Transport.
Hydrogen can also be used directly in automobiles, trucks, ships, and trains, either through fuel cell technology or internal combustion engines. In fuel cells, hydrogen generates electricity internally, which is then stored in a battery. In internal combustion, hydrogen is burned directly, emitting only water vapor. The transportation sector holds the greatest potential for carbon emissions reduction through hydrogen use.

Industry.
Thanks to its high calorific value and chemical properties, hydrogen can be deployed in energy-intensive industries such as oil refining, mining, cement, petrochemicals, and metallurgy. Beyond serving as an energy vector to optimize processes and improve energy efficiency, hydrogen is ideally suited to displace natural gas, resulting in significant CO₂ emissions reductions.

Raw material.
Traditionally, hydrogen is primarily used as a feedstock in the production of gasoline, polymers, solvents, ammonia, and fertilizers—processes that are currently associated with a high carbon footprint. However, when hydrogen is produced from renewable electricity sources, it becomes a priority for the future production of synthetic liquid fuels (such as methanol, kerosene, and jet fuel), green ammonia, and green fertilizers. This energy conversion process is referred to as Power-to-Liquids (P2L).
Photo by Richard Horvath on Unsplash
Hydrogen properties
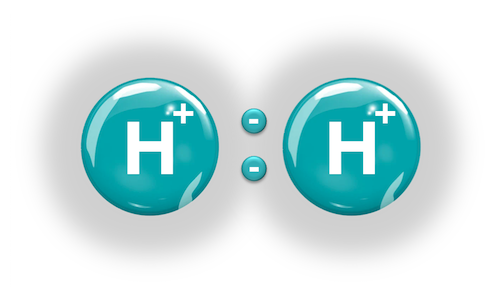
Hydrogen is a chemical element whose atomic structure has a proton (+) in its nucleus and an electron (-) in its orbital. It is represented by the symbol H and is located in group 1 and period 1 of the Periodic Table of Elements. When two hydrogen atoms are joined at standard temperature and pressure (25 ºC and 1 atm), the Hydrogen Molecule (H2) is formed and energy (heat) is released by the covalent bonding of electrons. Hydrogen has 3 natural isotopes: Protium (1H), Deuterium (2H), and Tritium (3H).
Hydrogen was discovered in 1766 by the scientist Henry Cavendish, who was the first to determine that hydrogen was a particular element that oxidized in the presence of oxygen (air) to produce water, and who recognized it as “flammable air”. In fact, its name means “water-forming” and comes from the Greek words “hydros“, water, and “genos“, generator. In 1925, the scientist Cecilia Payne-Gaposchkin determined that Hydrogen was the overwhelming constituent of stars, making it the most abundant element in the Universe.
Hydrogen Atom (H+):

Hydrogen Molecule (H2):
Physical properties:
| Atomic mass | 1.00797 g/mol |
| Molecular weight | 2.01594 g/mol |
| Density of gas | 0.0899 kg/m3 |
| Density of liquid | 70.79 kg/m3 |
| Melting point | 14.025 K (-259 ºC) |
| Boiling point | 20.268 K (-253 ºC) |
| Ignition point | 255 K (-18 ºC) |
| Flame temperature | 2318 – 2676 K (2045 – 2403 ºC) |
| Maximum flame speed | 346 cm/s |
| Ignition temperature with air | 803 K (530 ºC) |
| Low Heat Value (LHV) | 33.32 kWh/kg (119.97 MJ/kg) |
| High Heat Value (HHV) | 39.41 kWh/kg (141.89 MJ/kg) |
Hydrogen Fuel Quality Standards (ISO 14687:2019):
| Hydrogen Fuel Index (minimum) (a) | 99.97 % |
| Total Gases (maximum) (b) | 300 ppmv |
| Water (maximum) | 5 ppmv |
| Total Hydrocarbons (maximum) (c) | 2 ppmv |
| Methane (CH4) (maximum) | 100 ppmv |
| Oxygen (O2) (maximum) | 5 ppmv |
| Helium (He) (maximum) | 300 ppmv |
| Nitrogen (N2) (maximum) | 300 ppmv |
| Argon (Ar) (maximum) | 300 ppmv |
| Carbon dioxide (CO2) (maximum) | 2 ppmv |
| Carbon monoxide (CO) (maximum) (e) | 0.2 ppmv |
| Total Sulphur compounds (maximum) | 0.004 ppmv |
| Formaldehyde (maximum) (e) | 0.2 ppmv |
| Formic acid (maximum) (e) | 0.2 ppmv |
| Ammonia (NH3) (maximum) | 0.1 ppmv |
| Total Halogenated compounds (maximum) | 0.05 ppmv |
| Particulate size (maximum) | 10 μm |
| Particulate concentration (maximum @ NTP) (d) | 1μg/L |
Notes:
(a) The Hydrogen Fuel Index is the value obtained with the value of Total Gases measured in % subtracted from 100%.
(b) Total Gases = Sum of all impurities listed on the table except particulates.
(c) Total Hydrocarbons may exceed 2 ppmv only due to the presence of methane, provided that the total gases do not exceed 300 ppmv.
(d) μg/L @ NTP = micrograms per liter of hydrogen fuel at 0ºC and 1 atmosphere pressure.
(e) The sum of CO, HCHO and HCOOH shall not exceed 0.2 μmol/mol.
Source:
International Organization for Standardization (ISO). “ISO 14687:2019. Hydrogen fuel quality — Product specification”
Available on: https://h2tools.org/hyarc/hydrogen-data/hydrogen-fuel-quality-standards
Contact us
E-mail: contact@redlac-h2.org
